Quality Management Systems
- Input from QMS stakeholders is crucial during QMS design in order to maximize QMS-effectiveness and stakeholder “buy-in”.
- QMS operators need user-friendly turnkeys to unlock and efficiently run the system.
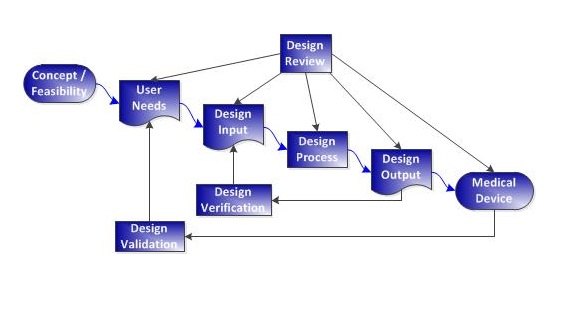
- Truly effective QMS processes are based on gold-standard practices that functionally address the letter and intent of QMS regulations and standards. It’s not enough to just read the regulations and copy them into procedures.
- Procedures should be written to simultaneously meet FDA and ISO 13485 requirements.
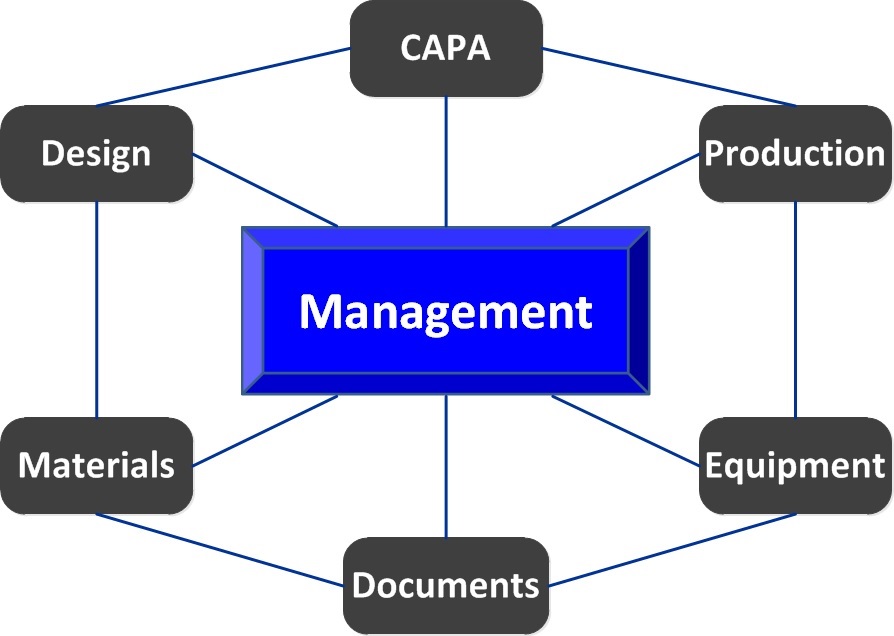
- Interdependent subsystems must be seamlessly integrated (like Risk Management and Corrective Action) to assure systemic continuity.
- QMS processes need to be strategically installed in the right order to reduce everyone’s frustration and to avoid wasted time.
These are some of the core principles that ComplianceAcuity uses when we assist you with your FDA or ISO 13485 QMS. Click on a link below to review the QMS solutions about which ComplianceAcuity is most passionate:
Testimonials
-
Director, Regulatory Affairs and Quality Assurance, Class II devices
-
Director of Development and Manufacturing Operations - Class II device Manufacturer
-
President & CEO, Class II device Manufacturer
-
Director of Operations – Class III device Manufacturer & Distributor
-
Director, Regulatory Affairs & Quality Assurance, Class II devices
-
Customer Service Supervisor – Class II & III device Manufacturer
-
Director of Operations – Class II and III device Manufacturer
-
President & Founder – Virtual Manufacturer of Class II devices
-
Director of Operations – Class II and III device Manufacturer
-
Director of Operations – Class III device Manufacturer & Distributor
-
Director of Operations – Class III device Manufacturer & Distributor
-
Quality Assurance Manager – Class II and III device Manufacturer
-
Director of Operations – Class II and III device Manufacturer
-
Sr. QA Manager, Design & Development
-
Quality Assurance Manager – Class II and III device Manufacturer
-
QA Director and VP of R&D – Virtual Manufacturer of Class III implantable devices
-
Business Development – Class II Device Virtual Manufacturer
-
QA Manager – Class II device Manufacturer
-
Sr. Quality Engineer – Class II & III device Manufacturer
-
Sr. QA Manager, Design & Development
-
Director of Operations – Class III device Manufacturer & Distributor
-
Director of Operations – Class III device Manufacturer & Distributor
-
Sr. QA Manager, Design & Development
-
Director of Operations – Class II and III device Manufacturer
-
Manager, Quality Systems – Class II & III device Manufacturer
-
Quality Assurance Manager – Class II and III device Manufacturer
-
QA Manager – Class II device Manufacturer
-
Director of Operations – Class II and III device Manufacturer
-
QA Manager – Class II and III device Manufacturer
-
Sr. QA Manager, Design & Development
-
Director of Operations – Class II and III device Manufacturer
-
Director of Quality – Class II & III device Manufacturer
-
Office Manager - Class II Software Device